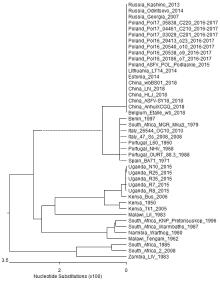
| [1] |
URBANO A C, FERREIRA F. Role of the DNA-binding protein pA104R in ASFV genome packaging and as a novel target for vaccine and drug development[J]. Vaccines, 2020, 8(4):585.
doi: 10.3390/vaccines8040585
|
| [2] |
ALONSO C, BORCA M, DIXON L, et al. ICTV virus taxonomy profile: Asfarviridae[J]. The Journal of General Virology, 2018, 99(5):613-614.
doi: 10.1099/jgv.0.001049
|
| [3] |
WANG F X, ZHANG H, HOU L N, et al. Advance of African swine fever virus in recent years[J]. Research in Veterinary Science, 2021, 136:535-539.
doi: 10.1016/j.rvsc.2021.04.004
pmid: 33882382
|
| [4] |
KEβLER C, FORTH J H, KEIL G M, et al. The intracellular proteome of African swine fever virus[J]. Scientific Reports, 2018, 8(1):1-9.
|
| [5] |
孙茂文, 王涛, 孙元, 等. 非洲猪瘟病毒的免疫逃逸策略[J]. 微生物学报, 2021, 61(2):249-262.
|
| [6] |
JANCOVICH J K, CHAPMAN D, HANSEN D T, et al. Immunization of pigs by DNA prime and recombinant vaccinia virus boost to identify and rank African swine fever virus immunogenic and protective proteins[J]. Journal of Virology, 2018, 92(8):e02219-17.
|
| [7] |
ALEJO A, MATAMOROS T, GUERRA M, et al. A proteomic atlas of the African swine fever virus particle[J]. Journal of Virology, 2018, 92(23):e01293-18.
|
| [8] |
ZHAO G H, LI T T, LIU X M, et al. African swine fever virus cysteine protease pS273R inhibits pyroptosis by noncanonically cleaving gasdermin D[J]. Journal of Biological Chemistry, 2022, 298(1):101480.
doi: 10.1016/j.jbc.2021.101480
|
| [9] |
LI G B, LIU X X, YANG M Y, et al. Crystal structure of African swine fever virus pS273R protease and implications for inhibitor design[J]. Journal of Virology, 2020, 94(10):e02125-19.
|
| [10] |
何健, 石建州, 刘阳坤, 等. 非洲猪瘟病毒研究进展[J]. 南阳师范学院学报, 2022, 21(1):55-62.
|
| [11] |
石建州, 何健, 刘阳坤, 等. 非洲猪瘟疫苗研究进展[J]. 中国兽医学报, 2022, 42(5):1057-1065,1076.
|
| [12] |
杜倩倩, 张智慧, 廉政, 等. 新型冠状病毒PLpro蛋白酶结构与功能的生物信息学分析[J]. 病毒学报, 2021, 37(1):43-51.
|
| [13] |
陈慧, 陈玉梅, 周怡, 等. 人WDR70蛋白结构与功能的生物信息学预测[J]. 井冈山大学学报(自然科学版), 2020, 41(2):32-38.
|
| [14] |
石姚黄, 王程媛, 梁晶莹, 等. 大鼠胰岛素增强子结合蛋白ISL1的生物信息学分析[J]. 井冈山大学学报(自然科学版), 2016, 37(5):29-35.
|
| [15] |
翟俊琼, 吴亚江, 代军威, 等. 东北虎γ-干扰素基因的克隆、生物信息学分析及其在毕赤酵母中的表达[J]. 中国畜牧兽医, 2021, 48(1):72-82.
|
| [16] |
王俊娟, 李欣芮, 陈成, 等. 花生主要过敏原Ara h 1线性B细胞表位的预测及鉴定[J]. 食品科学, 2021, 42(17):106-112.
|
| [17] |
王宇, 李建云, 武健, 等. 绵羊肺腺瘤病毒Gag蛋白的生物信息学分析及抗原表位预测[J]. 中国病原生物学杂志, 2021, 16(5):502-506.
|
| [18] |
王彦伟, 王孟月, 张素玲, 等. 非洲猪瘟病毒pS273R蛋白酶的可溶性表达与胞外活性鉴定[J]. 病毒学报, 2020, 36(5):879-884.
|
| [19] |
赵改红, 李婷婷, 张涛清, 等. 非洲猪瘟病毒pS273R蛋白的原核表达及其多克隆抗体的制备[J]. 中国畜牧兽医, 2021, 48(11):4154-4161.
|