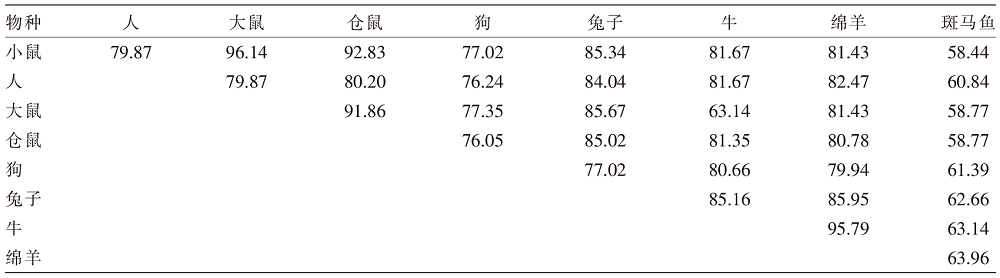
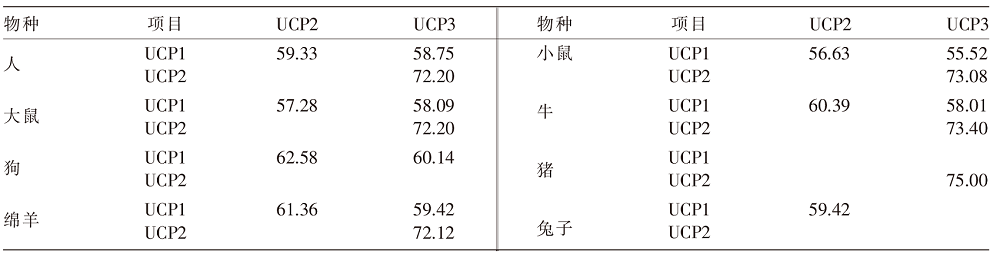
Animal Husbandry and Feed Science ›› 2023, Vol. 44 ›› Issue (5): 22-29.doi: 10.12160/j.issn.1672-5190.2023.05.004
• Basic Research • Previous Articles Next Articles
Recent Advances in Cold-inducible Proteins and Related Molecules in Animals
WANG Yanqin1,MENG Ningsheng1,HAN Na1,ZHI Yu2
- 1. Jining Normal College,Ulanqab 012000,China
2. Inner Mongolia Academy of Agricultural and Animal Husbandry Sciences,Hohhot 010031,China
-
Received:2023-03-13Online:2023-09-30Published:2023-11-14
CLC Number:
Cite this article
WANG Yanqin, MENG Ningsheng, HAN Na, ZHI Yu. Recent Advances in Cold-inducible Proteins and Related Molecules in Animals[J]. Animal Husbandry and Feed Science, 2023, 44(5): 22-29.
share this article
| [1] | BERTHOLET A M, KIRICHOK Y. The mechanism FA-dependent H+ transport by UCP1[J]. Handbook of Experimental Pharmacology, 2019, 251:143-159. |
| [2] |
HOU L J, XIE M Y, CAO L B, et al. Browning of pig white preadipocytes by co-overexpressing pig PGC-1α and mice UCP1[J]. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 2018, 48(2): 556-568.
doi: 10.1159/000491885 |
| [3] |
TALBOT D A, DUCHAMP C, REY B, et al. Uncoupling protein and ATP/ADP carrier increase mitochondrial proton conductance after cold adaptation of king penguins[J]. The Journal of Physiology, 2004, 558(1): 123-135.
doi: 10.1113/jphysiol.2004.063768 |
| [4] | CRISCUOLO F, GONZALEZ-BARROSO M D, LE MAHO Y, et al. Avian uncoupling protein expressed in yeast mitochondria prevents endogenous free radical damage[J]. Proceedings Biological Sciences, 2005, 272(1565): 803-810. |
| [5] |
HU F, LI C, YE Y F, et al. PARP12 is required for mitochondrial function maintenance in thermogenic adipocytes[J]. Adipocyte, 2022, 11(1): 379-388.
doi: 10.1080/21623945.2022.2091206 pmid: 35916471 |
| [6] |
JUSTESEN S, HAUGEGAARD K V, HANSEN J B, et al. The autocrine role of FGF21 in cultured adipocytes[J]. The Biochemical Journal, 2020, 477(13): 2477-2487.
doi: 10.1042/BCJ20200220 |
| [7] |
XU L, LI D D, LI H R, et al. Suppression of obesity by melatonin through increasing energy expenditure and accelerating lipolysis in mice fed a high-fat diet[J]. Nutrition and Diabetes, 2022, 12(1): 42.
doi: 10.1038/s41387-022-00222-2 |
| [8] |
ZININGA T, RAMATSUI L, SHONHAI A. Heat shock proteins as immunomodulants[J]. Molecules, 2018, 23(11): 2846.
doi: 10.3390/molecules23112846 |
| [9] |
PARK E, COCKREM J F, HAN K H, et al. Stress-induced activation of ovarian heat shock protein 90 in a rat model of polycystic ovary syndrome[J]. The Journal of Obstetrics and Gynaecology Research, 2012, 38(2): 396-407.
doi: 10.1111/j.1447-0756.2011.01705.x pmid: 22176470 |
| [10] |
YUN C W, KIM H J, LIM J H, et al. Heat shock proteins: Agents of cancer development and therapeutic targets in anti-cancer therapy[J]. Cells, 2019, 9(1): 60.
doi: 10.3390/cells9010060 |
| [11] |
LIU X T, LI S, ZHAO N, et al. Effects of acute cold stress after intermittent cold stimulation on immune-related molecules, intestinal barrier genes, and heat shock proteins in broiler ileum[J]. Animals, 2022, 12(23): 3260.
doi: 10.3390/ani12233260 |
| [12] |
JOO S Y, PARK M J, KIM K H, et al. Cold stress aggravates inflammatory responses in an LPS-induced mouse model of acute lung injury[J]. International Journal of Biometeorology, 2016, 60(8): 1217-1225.
doi: 10.1007/s00484-015-1116-5 |
| [13] |
TRINGALI G, FARRACE S, RAGAZZONI E, et al. Circulating interleukin-1-beta levels after acute and prolonged exposure to low temperatures:Human and rat studies[J]. Neuroimmunomodulation, 2000, 7(4): 177-181.
doi: 10.1159/000026436 |
| [14] |
REN J Y, LIU C P, ZHAO D, et al. The role of heat shock protein 70 in oxidant stress and inflammatory injury in quail spleen induced by cold stress[J]. Environmental Science and Pollution Research, 2018, 25(21): 21011-21023.
doi: 10.1007/s11356-018-2142-8 |
| [15] |
XU B, ZANG S C, LI S Z, et al. HMGB1-mediated differential response on hippocampal neurotransmitter disorder and neuroinflammation in adolescent male and female mice following cold exposure[J]. Brain, Behavior, and Immunity, 2019, 76:223-235.
doi: S0889-1591(18)30510-5 pmid: 30476565 |
| [16] |
MAO L P, JIAO Y, XIANG J H, et al. Cold-inducible RNA-binding protein migrates from the nucleus to the cytoplasm under cold stress in normal human bronchial epithelial cells via TRPM8-mediated mechanism[J]. Annals of Translational Medicine, 2021, 9(18): 1470.
doi: 10.21037/atm |
| [17] |
LIU J Q, HU T Y, DIAO K Y, et al. Cold stress promotes IL-33 expression in intestinal epithelial cells to facilitate food allergy development[J]. Cytokine, 2020, 136:155295.
doi: 10.1016/j.cyto.2020.155295 |
| [18] |
ZHU X Z, BÜHRER C, WELLMANN S. Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold[J]. Cellular and Molecular Life Sciences, 2016, 73(20): 3839-3859.
doi: 10.1007/s00018-016-2253-7 pmid: 27147467 |
| [19] |
YANG J, WU H Q, XIE W Y, et al. Cold-inducible RNA-binding protein mediates airway inflammation and mucus hypersecretion through a post-transcriptional regulatory mechanism under cold stress[J]. The International Journal of Biochemistry and Cell Biology, 2016, 78:335-348.
doi: 10.1016/j.biocel.2016.07.029 |
| [20] |
LIU Y Y, XUE N Y, ZHANG B X, et al. Cold stress induced liver injury of mice through activated NLRP3/caspase-1/GSDMD pyroptosis signaling pathway[J]. Biomolecules, 2022, 12(7): 927.
doi: 10.3390/biom12070927 |
| [21] |
KIM S Y, BAN H J, LEE S, et al. Regulation of CIRP by genetic factors of SP1 related to cold sensitivity[J]. Frontiers in Immunology, 2022, 13:994699.
doi: 10.3389/fimmu.2022.994699 |
| [22] |
ROILO M, KULLMANN M K, HENGST L. Cold-inducible RNA-binding protein (CIRP) induces translation of the cell-cycle inhibitor p27Kip1[J]. Nucleic Acids Research, 2018, 46(6): 3198-3210.
doi: 10.1093/nar/gkx1317 pmid: 29361038 |
| [23] |
ZHOU K, CUI S T, DUAN W, et al. Cold-inducible RNA-binding protein contributes to intracerebral hemorrhage-induced brain injury via TLR4 signaling[J]. Brain and Behavior, 2020, 10(6): e01618.
doi: 10.1002/brb3.v10.6 |
| [24] |
TONG G, ENDERSFELDER S, ROSENTHAL L M, et al. Effects of moderate and deep hypothermia on RNA-binding proteins RBM3 and CIRP expressions in murine hippocampal brain slices[J]. Brain Research, 2013, 1504:74-84.
doi: 10.1016/j.brainres.2013.01.041 pmid: 23415676 |
| [25] |
LOGAN S M, STOREY K B. Cold-inducible RNA-binding protein Cirp, but not Rbm3, may regulate transcript processing and protection in tissues of the hibernating ground squirrel[J]. Cell Stress and Chaperones, 2020, 25(6): 857-868.
doi: 10.1007/s12192-020-01110-3 |
| [26] |
LIU Y, LIU P, HU Y J, et al. Cold-induced RNA-binding protein promotes glucose metabolism and reduces apoptosis by increasing AKT phosphorylation in mouse skeletal muscle under acute cold exposure[J]. Frontiers in Molecular Biosciences, 2021, 8:685993.
doi: 10.3389/fmolb.2021.685993 |
| [27] |
HU Y J, LIU Y, QUAN X, et al. RBM3 is an outstanding cold shock protein with multiple physiological functions beyond hypothermia[J]. Journal of Cellular Physiology, 2022, 237(10): 3788-3802.
doi: 10.1002/jcp.30852 pmid: 35926117 |
| [28] |
XIA W L, SU L B, JIAO J W. Cold-induced protein RBM3 orchestrates neurogenesis via modulating Yap mRNA stability in cold stress[J]. The Journal of Cell Biology, 2018, 217(10): 3464-3479.
doi: 10.1083/jcb.201801143 |
| [29] |
FENG J G, PAN W, YANG X L, et al. RBM3 increases cell survival but disrupts tight junction of microvascular endothelial cells in acute lung injury[J]. Journal of Surgical Research, 2021, 261:226-235.
doi: 10.1016/j.jss.2020.12.041 pmid: 33460967 |
| [30] |
USHIO A, ETO K. RBM3 expression is upregulated by NF-κB p65 activity, protecting cells from apoptosis, during mild hypothermia[J]. Journal of Cellular Biochemistry, 2018, 119(7): 5734-5749.
doi: 10.1002/jcb.26757 pmid: 29388696 |
| [31] |
HETTINGER Z R, CONFIDES A L, VANDERKLISH P W, et al. Skeletal muscle RBM3 expression is associated with extended lifespan in Ames dwarf and calorie restricted mice[J]. Experimental Gerontology, 2021, 146:111214.
doi: 10.1016/j.exger.2020.111214 |
| [32] |
YUAN X, ZHANG J, MA T T, et al. Expression regulation of cold-inducible protein RBM3 by FAK/Src signaling for neuroprotection against rotenone under mild hypothermia[J]. Biochemical and Biophysical Research Communications, 2021, 534:240-247.
doi: 10.1016/j.bbrc.2020.11.105 pmid: 33272569 |
| [33] |
LIU Y, XU B, HU Y J, et al. O-GlcNAc/Akt pathway regulates glucose metabolism and reduces apoptosis in liver of piglets with acute cold stress[J]. Cryobiology, 2021, 100:125-132.
doi: 10.1016/j.cryobiol.2021.02.008 |
| [34] |
LIU Y, SHI H Z, HU Y J, et al. RNA binding motif protein 3 (RBM3) promotes protein kinase B (AKT) activation to enhance glucose metabolism and reduce apoptosis in skeletal muscle of mice under acute cold exposure[J]. Cell Stress and Chaperones, 2022, 27(6): 603-618.
doi: 10.1007/s12192-022-01297-7 |
| [35] |
FUJITA T, LIU Y, HIGASHITSUJI H, et al. Involvement of TRPV3 and TRPM8 ion channel proteins in induction of mammalian cold-inducible proteins[J]. Biochemical and Biophysical Research Communications, 2018, 495(1): 935-940.
doi: S0006-291X(17)32315-X pmid: 29175331 |
| [36] |
JACKSON T C, KOTERMANSKI S E, KOCHANEK P M. Infants uniquely express high levels of RBM3 and other cold-adaptive neuroprotectant proteins in the human brain[J]. Developmental Neuroscience, 2018, 40(4): 325-336.
doi: 10.1159/000493637 pmid: 30399610 |
| [37] |
BASTIDE A, PERETTI D, KNIGHT J R P, et al. RTN3 is a novel cold-induced protein and mediates neuroprotective effects of RBM3[J]. Current Biology, 2017, 27(5): 638-650.
doi: S0960-9822(17)30082-9 pmid: 28238655 |
| [38] |
SCHAGATAY F, DIAMANT K, LIDEN M, et al. Serum concentration of extracellular cold-inducible RNA-binding protein is associated with respiratory failure in COVID-19[J]. Frontiers in Immunology, 2022, 13:945603.
doi: 10.3389/fimmu.2022.945603 |
| [1] | GAO Yu, YIN Jun. Research Progress of Hair Follicle Morphogenesis and Related Signaling Pathways in Mammals [J]. Animal Husbandry and Feed Science, 2023, 44(2): 66-71. |
| [2] | ZHANG Tong, ZHANG Yu, WANG Shi-nong, WANG Jia-ru, LUO Ying-hua, JIN Cheng-hao. Research Progress on Anti-tumor Pharmacological Effects and Mechanism of Isoorientin [J]. Animal Husbandry and Feed Science, 2019, 40(9): 86-88. |
| [3] | WANG Dong-yang, Baotuya. Research Progress on Beta-defensins in Female Mammalian Reproductive System [J]. Animal Husbandry and Feed Science, 2019, 40(6): 30-33. |
| [4] | . Effect of Kinetin on T Lymphocyte Subsets in Peripheral Blood and Immunity of Aging Mice [J]. Animal Husbandry and Feed Science, 2016, 37(6): 23-23. |
| [5] | . Research on Mammalian XY Sperm Separation Technology [J]. Animal Husbandry and Feed Science, 2014, 35(7): 56-56. |
| [6] | . Research Progress of Apoptosis Molecular Mechanism [J]. Animal Husbandry and Feed Science, 2013, 34(4): 80-80. |
| [7] | . Study on the Relationship between Animal Heat Production and Metabolic Weight of Zhongwei Goat Lactating Ewes [J]. Animal Husbandry and Feed Science, 2013, 34(11): 26-26. |
| [8] | . Study on the Cryopreservation Method of Holstein Cow Mammary Epithelial Cells [J]. Animal Husbandry and Feed Science, 2011, 32(9): 65-65. |
| [9] | . Toxicity Effect of Estrogens from Environment on Male Reproductive [J]. Animal Husbandry and Feed Science, 2011, 32(3): 81-81. |
| [10] | . Research Progress in Parthenogenetic Activation of Mammalian Oocytes [J]. Animal Husbandry and Feed Science, 2010, 31(11): 14-14. |
| [11] | . Research Advances on Apoptosis [J]. Animal Husbandry and Feed Science, 2010, 31(11): 17-17. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||